The 7 major rules of the FDA's Food Safety Modernization Act (FSMA)

We live in an age where constant change is the norm in the global food system, with a series of questions and issues surfacing with each novelty.
A decade ago, the FDA's Food Safety Modernization Act (FSMA) was created and enforced to mitigate this situation and establish clear safety guidelines in the United States.
Every consumer out there, including you and me, expects all parties involved—from the farm through to the distributors and retail food outlets—to do their best to bring safe and high-quality food products on the market. And that’s where FSMA regulations come in to save the day (and the food).
How the FDA's Food Safety Modernization Act (FSMA) came into being
Just consider one of the many devastating outbreaks born of neglectful food handling practices in recent years. In Oregon and Washington in 2015, many people fell deathly ill due to E. Coli outbreaks in local Chipotle chains, and that’s just the tip of the iceberg, according to the Centers for Disease Control and Prevention (CDC).
But it's situations like these that have compelled the Food and Drug Administration (FDA) to create and help implement general safety standards that, once adopted extensively by the food industry, should reduce the risk of contamination.
Thus, the Food Safety Modernization Act was signed into law by President Obama on January 4, 2011, launching a new era of food safety regulations.

The FSMA aims to transform the United States' food safety system by focusing on preventing foodborne illnesses rather than just responding to them after an outbreak occurs.
This resolution requires a multitude of standards to be met and procedures to be followed in order to comply. We’re here as your partners in regulatory compliance for visitor management to give you an overview of everything you need to know to be prepared.
The 7 major FSMA rules you must know
Since September 2015, the FDA has published 7 FSMA final rules for the implementation of modern food safety prevention practices by those who grow, process, transport, and store food.

Let’s look into each of these rules one by one and determine the main requirements for compliance, to whom it applies, and the FSMA compliance dates.
- Standards for the growing, harvesting, packing, and holding of produce for human consumption
- Mitigation strategies to protect food against intentional adulteration
- Current good manufacturing practice and hazard analysis and risk-based preventative controls for food for humans and animal
- Sanitary transportation of human and animal food
- Accredited third-party certification bodies to conduct food safety audits and issue certifications
- Foreign Supplier Verification Program (FSVP) for importers of food for humans and animals
- Voluntary Qualified Importer Program (VQIP)
1. Standards for the growing, harvesting, packing, and holding of produce for human consumption
The Produce Safety Rule applies to covered farms and “establishes science-based minimum standards for the safe growing, harvesting, packing, and holding of fruits and vegetables grown for human consumption.”
However, these standards don’t apply to produce that is rarely consumed raw, produce for personal or on-farm consumption, or produce that is not a raw agricultural commodity.
Also, the main requirements involve the following:
- Microbiological standards for agricultural water and biological soil amendments of animal origin
- Monitoring and correction activities for domesticated and wild animals
- Measures for preventing the contamination of sprouts
- CGMP-like requirements for worker health and hygiene, equipment, tools, and buildings
2. Mitigation strategies to protect food against intentional adulteration
This rule is concerned with “preventing intentional adulteration from acts intended to cause wide-scale harm to public health, including acts of terrorism targeting the food supply.”
Its requirements apply to both domestic and foreign companies required to register with the FDA as food facilities. However, the rule doesn’t cover very small businesses and farms.
All the facilities covered by the Intentional Adulteration (IA) rule must develop and implement a written food defense plan that includes an analysis of vulnerabilities, implementation of mitigation strategies, procedures for food defense monitoring, corrective actions, and verification.
Compliance with this rule will be verified starting March 2021 with routine inspections of all small businesses.
3. Current good manufacturing practice and hazard analysis and risk-based preventive controls for food for humans and animals
For human food
This rule applies to food facilities - registered with section 415 of the Food, Drug, & Cosmetic Act - and requires them to have a food safety plan implemented that includes an analysis of hazards and risk-based preventive controls to minimize or prevent the identified threats.
The concerned facilities are required to create and implement a food safety system that includes a number of points:
- Hazard analysis: identifying any known or reasonably foreseeable biological, chemical, and physical hazards
- Preventive controls: establishing preventive controls - such as process controls, food allergen controls, and sanitation controls - to address hazards that occur in the manufactured products
- Oversight and management of preventive controls
- Supply chain program: implementing a risk-based supply chain program
- Recall plan
For animal food
The requirements of this rule also apply to animal food facilities and oblige them to have a food safety plan in place, as in the case of the previous regulation.
Apart from the safety plan, which is similar to the food facilities producing human food, covered facilities must follow Current Good Manufacturing Practices (CGMPs) for animal food production, which have been created while taking into consideration the unique aspects of the animal food industry.
4. Sanitary Transportation of Human and Animal Food
The main goal of this rule is to keep food safe from contamination during transportation by preventing issues such as failure to properly refrigerate and protect food and inadequate cleaning of vehicles between loads.
Its requirements apply to shippers, loaders, carriers by motor or rail vehicle, and receivers involved in transporting human and animal food. Nonetheless, these requirements don’t apply to transportation by ship or air due to limitations in the law.
On the whole, the rule establishes several requirements addressing the following:
- the design and maintenance of vehicles and transportation equipment
- measures taken during transportation to ensure food safety
- training of carrier personnel in sanitary transportation practices and documentation of the training
- maintenance of records of written procedures, agreements, and training
5. Accredited third-party certifications
The rule establishes “the framework, procedures, and requirements for accreditation bodies seeking recognition by the FDA, as well as requirements for third-party certification bodies seeking accreditation.”
These entities will be able to conduct food safety audits and certify that foreign food facilities and food produced by such facilities follow FDA food safety guidelines.
Also, the FDA published a public registry of recognized accreditation bodies on their website, as well as a list of accredited third-party certification bodies.
FSMA mentions two uses for certifications under this program:
- to help establish eligibility for participation in the Voluntary Qualified Importer Program (VQIP), and,
- to prevent potentially harmful food from reaching U.S. consumers.
6. Foreign Supplier Verification Program (FSVP)
The Foreign Supplier Verification Program regulation applies to importers of food (human and animal) into the United States. It requires said importers to perform certain risk-based activities to check that imported food has been produced in a manner that meets applicable U.S. safety standards.
According to this rule, an importer is defined as “the U.S. owner or consignee of an article of food that is being offered for import into the United States. If there is no U.S. owner or consignee at the time of U.S. entry, the importer is the U.S. agent or representative of the foreign owner or consignee at the time of entry, as confirmed in a signed statement of consent.”
Moreover, importers are responsible for actions such as:
- Determining known or reasonably foreseeable hazards for each food
- Evaluating the risk posed by a food, based on the hazard analysis, and the foreign supplier’s performance
- Using that evaluation of the risk posed by an imported food and the supplier’s performance to approve suppliers and determine appropriate supplier verification activities
- Conducting supplier verification activities
- Conducting corrective actions
7. Voluntary Qualified Importer Program (VQIP)
This is a fee-based voluntary program for expedited review and import entry of human and animal foods into the United States.
Simply put, participating importers that meet eligibility criteria, and pay a user fee, can import their products to the U.S. with greater speed and predictability. The benefits of this are clear for both importers and consumers alike.
The FDA does a good job of answering the most frequently asked questions:
- What are the benefits of participating in VQIP?
- What are the eligibility criteria to participate in VQIP?
- Why is there a user fee to participate in VQIP?
- How do I apply to VQIP?
- What information do I need to complete the VQIP application?
The answers can be found here.
How does Proxyclick help you comply with the FSMA?
Now that we've taken a closer look at the major rules of FSMA, let's discuss exactly what this means for your visitor management.
The ultimate goal of the FDA's Act is to make food handling safer in the United States and prevent food-related safety issues from sprouting up in the U.S. food supply, which means that visitors and contractors entering your production sites and premises must be handled with care.
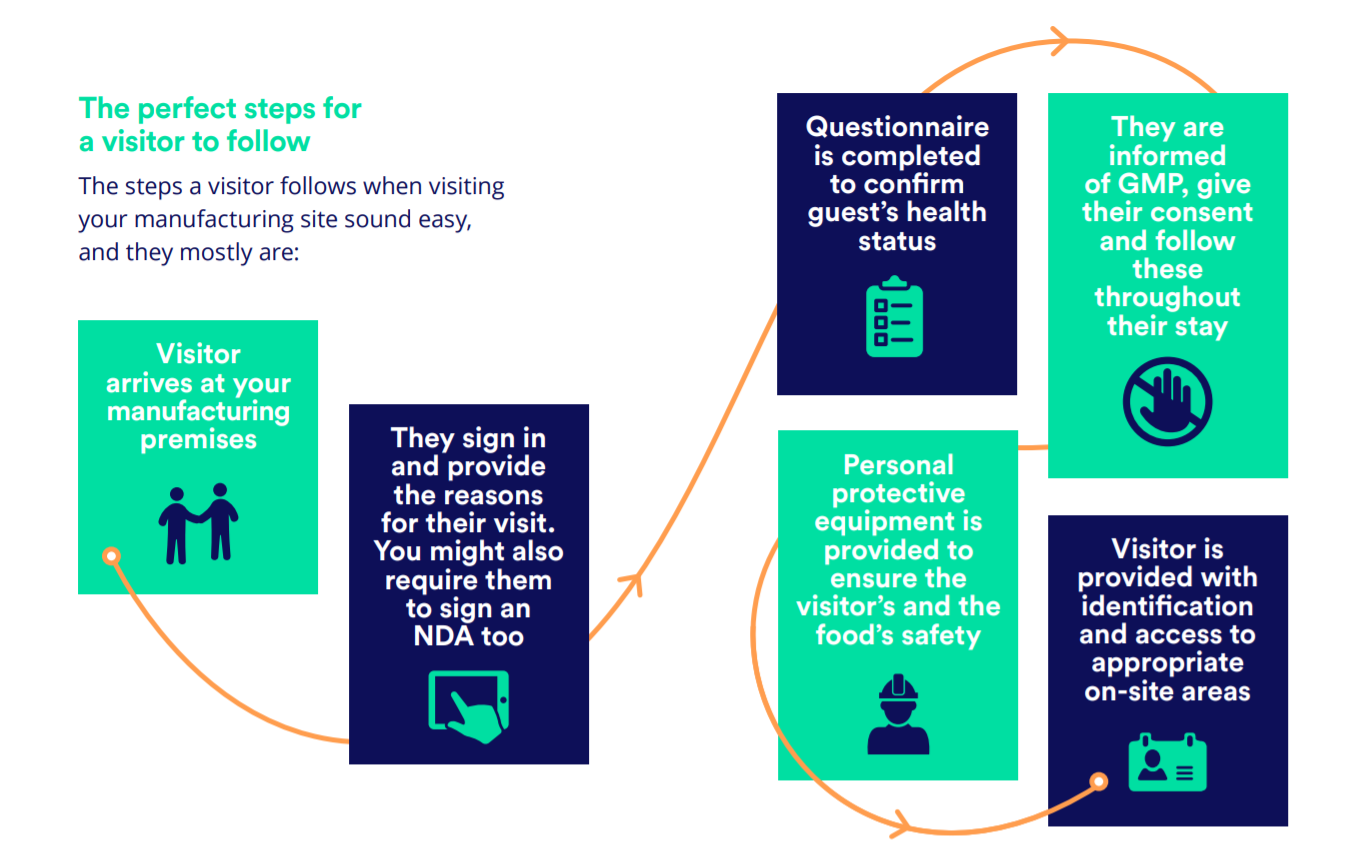
When a visitor or contractor arrives at your food manufacturing site, it’s your team's responsibility to ensure all the safety requirements are met. At the same time, your external visitors should feel welcomed and safe.
With key technologies and the right visitor management system in place, this is wholly possible:
- Document and create custom workflows according to Q&As for health and safety guidelines (Download our sample health questionnaire)
- Enforce mandatory pre-registration of all visitors and contractors
- Screen all visitors against watchlists to deny access to restricted or banned individuals
- Send security alerts if unauthorized access is attempted
- Ensure visitors follow Good Manufacturing Practice (GMP) and provide their consent through a streamlined process
- Improve the provision of Personal Protective Equipment (PPE) for visitors and ensure they only have access to appropriate areas on your site
- Collect digital signatures on-site regulations and agreements
- Print detailed and custom visitor badges for identification and authorization
- Depend on secure cloud storage of your visitor data
- Track and export visitor histories and their precise movements for reporting and audit purposes
In conclusion
The Food Safety Modernization Act is here to help strengthen the global food chain and regulations for general safety.
Once you've determined which of the requirements mentioned above apply to your business, you’ll be able to implement the necessary procedures and safety measures for FSMA compliance. Also, the compliance dates for these 7 rules vary, in part, according to the size of the business involved. You can check them out, here.
Automating this process by using a cloud-based visitor management system can help you save time and enable you to focus your efforts on other needs of your business. With compliance costs projected to more than double by 2022, there's no time like the present to get ahead of the curve.
Want to learn more about how Proxyclick helps with covering the bases when it comes to audits and regulations? Contact us directly, or book a demo with one of our experts below.
***
Disclaimer: The information presented above is not legal advice, is not to be acted on as such, may not be current, and is subject to change without notice. You should seek professional legal counsel before taking any action.



